
CQV Utilities
Commissioning, Qualification, and Validation (CQV) in the life science pharmaceutical and medical device industries define a detailed and science-based process for the specification, design, and verification of new equipment or systems into a production pipeline.
All Facilities, Utilities and Equipment (FUE) need to be Commissioned AND Qualified. All Utilities like Compressed Air System, Nitrogen Plant and Distribution System, Pure Steam Generation System and WFI System.
All Utilities will be Qualified based on following Guidelines
- ISPE Baseline Guide 5 “Commissioning and Qualification (Second Edition)”
- U.S. FDA “Process Validation: General Principles and Practices”
- ICH Q9 “Quality Risk Management”
- ASTM E 2500 “Standard Guide for Specification, Design, and Verification of Pharmaceutical and Biopharmaceutical Manufacturing Systems and Equipment”
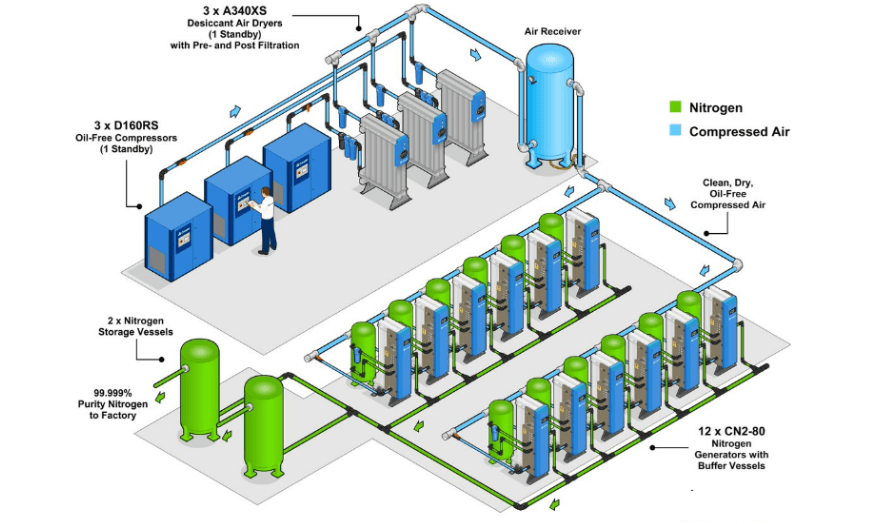
Compressed Air System
CQV Execution Plan
- Develop the Validation Master Plan.
- Complete Gap and Risk Assessments.
- Perform Pre-Startup Safety Reviews.
- Generate, perform, and document the Factory Acceptance Test (FAT) and Site Acceptance Test (SAT).
- Execute a System Impact Assessment (SIA).
- Provide Installation and operational qualifications (IQ/OQ) for Building Management System (BMS) Systems and Environmental Monitoring Systems Development and assessments for maintenance programs.
- Detailed Operational Qualifications Process Validation (PV).
- SOP development and preventative maintenance implementation.
- Turnover documentation package preparation.
- Compile the CQV Issues Log.
- Finalize the project with a CQV Summary Report (CQVSR).
CQV Deliverables
- VMP
- URS
- FRS
- Commissioning (FAT,SAT)
- Risk Assessments
- Change Management
- PM/Calibration Program
- Qualification IQ/OQ/PQ
- SOP Development/ Training
- GMP release



 Copyright GAMP Services 2024. All Rights Reserved.
Copyright GAMP Services 2024. All Rights Reserved.