
Aseptic Facility Validation
Aseptic Facility & Filling Qualification
Aseptic food processing can produce high-quality packaged goods that are shelf-stable for long periods. However, if not properly produced and packaged, aseptic foods can be dangerous to the consumer. Consider these six steps to ensure product safety.
Aseptic Processing
Validation of the preparation, processing and packaging of Aseptic Processing .The aseptic process is designed and validated to inactivate all microorganisms that pose a threat to public health and those that cause spoilage. As a result, products are produced that are commercially sterile and can have a long shelf life.
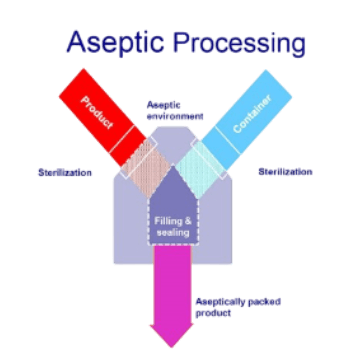
Sterilisation Techniques
There are four accepted and commercially available sterilisation techniques for high-speed rotary aseptic filling lines that can be applied to both PET and HDPE bottles as well as plastic caps, each one with different characteristics :
- Wet PAA-based technology sprays liquid Peroxyacetic Acid at high pressure and relatively low temperature. Light PET bottles can withstand Wet PAA treatment without significant shrinkage. This simple and robust system can also be applied in a similar way to flat caps, which are immersed in a liquid PAA sterilisation bath to distribute the sterilant on the cap surfaces and reach the desired killing rate. Rinsing with sterile water removes the last residuals of the PAA solution.
- Vapour PAA treatment uses small quantities of PAA in food-grade steam. This uses very small amounts of chemical but is more complicated as the temperature of the bottle must be closely monitored to ascertain the activation of the sterilising agent. Further, it may not be sufficiently effective when high sterilisation levels are required.
- H2O2 CHP container sterilisation uses a Hydrogen Peroxide vapour that condenses when it is injected into the bottle, causing micro drops to form on the internal walls. These are removed with hot air. H2O2 CHP treatment that is also used for foil sterilisation.
- H2O2 VHP (Vapourised Hydrogen Peroxide) is a dry sterilisation technique using H2O2 The bottles are heated before treatment to prevent the steam from condensing on the internal surfaces, minimising residue values. This technique can be easily applied to treat sterilisable flat and sport caps.
- To reduce the effect of heat on the bottles, some systems operate by sterilising the preform before blowing in a sterile environment, thereby minimising the amount of chemical required and preventing shrinkage. CHP and VHP processes are preferred for preform decontamination in aseptic lines. The CHP sterilisation process takes place before the oven: this implies an increase in costs and risks related to preventing the potential preform re-contamination inside the oven, the impossibility of getting the starting temperature of the preform under control and, consequently, the amount of H2O2 condensed inside the preform. The VHP treatment on preforms takes place after the oven: the oven heats the preform to around 100 deg C and is then treated with H2O2 vapour at 80-90 deg C. Once sterilised, the preform is blown in a sterile environment and then filled.


Following are six key considerations to ensure aseptic food products are safely produced:
1. Coordinating aseptic food processing operations
Aseptic processing and packaging consist of several operations that need to work safely concurrently, and any misstep can end in an unsafe product. Every part of the system—from the utilities, ingredient dosing, batching and mixing, to the control and data recorder—must be fit for purpose and perform as designed at all times. It is necessary to have a plan to validate, verify and control maintenance and changes made to each of these units to ensure the safety of the products.
2. Filing with the FDA
Each product must be filed with the FDA. The filing documents must contain these components:
- The thermal process,
- equipment sterilization and maintenance of sterility, filling and packaging machine validation,
- and final product, which has been validated using a combination of microbiological and physicochemical tests.
3. Testing your aseptic food process for quality assurance
While products made under the filed conditions are in principle safe, it is normal practice to test a few samples for positive release; the sampling program has to be agreed upon beforehand. Typically, a few samples are incubated in a hot box for seven to fifteen days to make sure there are no off-orders or observable package bloating. Even though the aseptic processes are validated, it is a good idea to have the in-house microbiology lab perform aerobic plate count (APC) and yeast/mold tests to rapidly detect defects in post-processing and package closures. If your product is low acid, then the QA tests should include mesophilic and thermophilic anaerobic spores, which are the hardest to eliminate and the ones that have the potential to produce Clostridium botulinum.
4. Monitoring for deviations
QA management should always review these batch or lot records as well as data on closure integrity to ensure that the CCP parameters are in the control. Document any deviations observed in the CCPs and apply the deviation plan. If this does occur, quarantine products and pursue different action or testing, such as performing a Root Cause Analysis (RCA) to determine corrective action.
5. Creating a product release procedure
Facilities should develop and implement a product release procedure outlining the responsibility and protocols for the release of products, including deviations management. This program also applies to the procedures for releasing quarantined or held product. These procedures might include outlining process measures that demonstrate that products are compliant with specified requirements or that products that do not meet the specifications are safely disposed.
6. Keeping proper records for aseptically processed foods
As the FDA saying goes, if you didn’t document it, it didn’t happen. Records for all products released should be maintained and show the product’s name and identification, confirmation of checks and what happened to the product, whether it was released, incubated or held. If a held product is released, that should also be documented, detailing how much of that product was held and why. Furthermore, regulation requires that these production records, either in the form of a secure electronic copy or an old-fashioned hard copy, are safely stored and easily accessible for at least three years.
A product release program, successfully followed, will only allow compliant products to be released to the market. And that means all your aseptic food and beverages will be the safest and highest quality they can be.
For INTEGRATED ASPETC OPERATION, There are 3 main components:
- Cleanroom & HVAC System with classification: Follow ISO 14644-3 for validation
- Sterilization System: Follow EN 285,ISO 11135,ISO 11137
- Aseptic Filling Line: Complete Documentation with EM Sampling and Smoke Study





 Copyright GAMP Services 2024. All Rights Reserved.
Copyright GAMP Services 2024. All Rights Reserved.