
Equipment
CQV Step for Equipment
- Focuses on Critical Design Elements (CDE’s) and Critical Process Parameters (CPP’s) which provide the ability to deliver Critical Quality Attributes (CQA’s)
- Provides guidance on how to apply the Quality Risk Management (QRM) process to C&Q, to establish Critical Design Elements (CDEs), which are subject to qualification
- Clarifies User Requirements, Design Review (DQ), and
- System Acceptance and Release
- Recommends an integrated C&Q approach, that begins at the Design Phase
- Clarifies Change Management processes required to support the C&Q process
- Recognizes current industry best practices
- Provides GEP documentation standards
CQV Risk Assessment
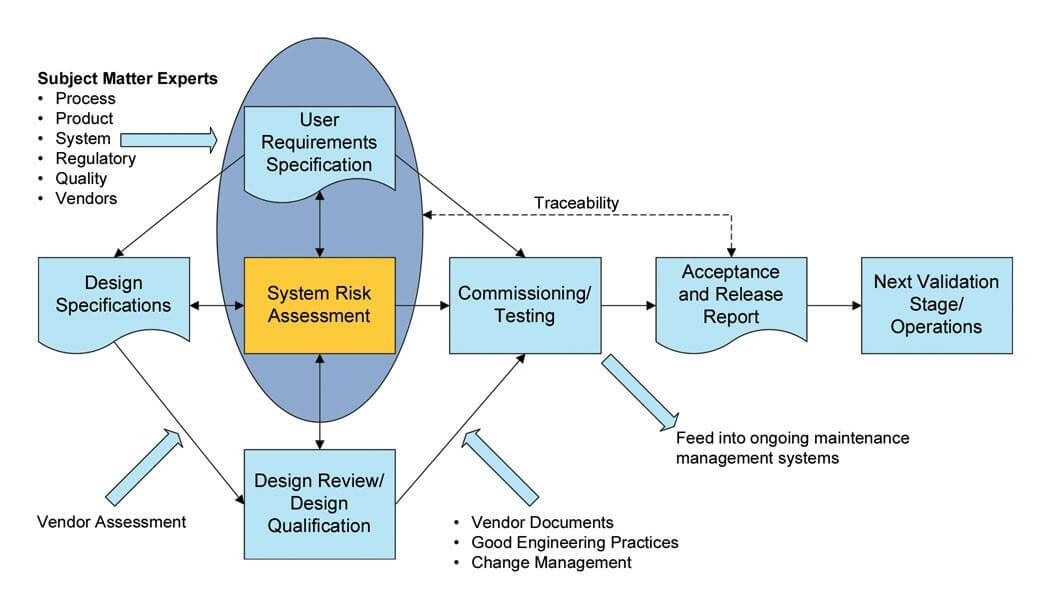
CQV For Sterile Plant Equipment :
- Part Washer
- Autoclave
- Rotary Autoclave
- Sterilizing Tunnel
- Lyophilizer
- Filling Line
- CIP/SIP SKID
- Isolator
- Fermentation & Bioreactor
- Packing Line
CQV For Formulation/API Plant Equipment :
- Reactors
- Compression Machines
- FBDs, FBEs
- RMG
- Blenders
- Spray dryer etc.
- Blister Pack
- Tablet Counting Machine
- Labelling Machine
- Check Weigher
- Labelling M/c etc.
CQV Deliverables
- VMP
- URS
- FRS
- Commissioning (FAT,SAT)
- Risk Assessments
- Change Management
- PM/Calibration Program
- Qualification IQ/OQ/PQ
- SOP Development/ Training
- GMP release
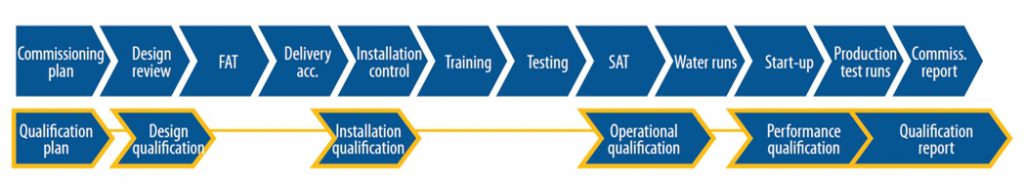
CQV Project Step
- Analyzing Project Scope
- Building a Team.
- Developing the Master Validation Plan (MVP)
- Commissioning
- Quipment Qualification
- Validation
- Continued Process Verification
- Maintaining a State of Compliance
We will cover other systems and processes such as validation for software, data integrity, computer systems, and so forth, which will be discussed in our upcoming blogs.
Here are some current CQV industry guideline references for further reading:
- FDA 21 CFR Part 210
- FDA 21 CFR Part 211,
- ISPE Baseline Guide 5 Commissioning and Qualification (Second Edition)
- ISPE GAMP 5, A Risk-Based Approach to Compliant GxP Computerized Systems
- The U.S. FDA Process Validation: General Principles and Practices
- ICH Q6A and Q6B Specifications
- ICH Q7, Good Manufacturing Practice
- ICH Q9 Quality Risk Management
- ICH Q10, Pharmaceutical Quality System
- ICH Q11, Development and Manufacture of Drug Substances
- ICH Q12, Lifecycle Management
- ASTM E 2500 Standard Guide for Specification, Design, and Verification of Pharmaceutical and Biopharmaceutical Manufacturing Systems and Equipment



 Copyright GAMP Services 2024. All Rights Reserved.
Copyright GAMP Services 2024. All Rights Reserved.