CSV
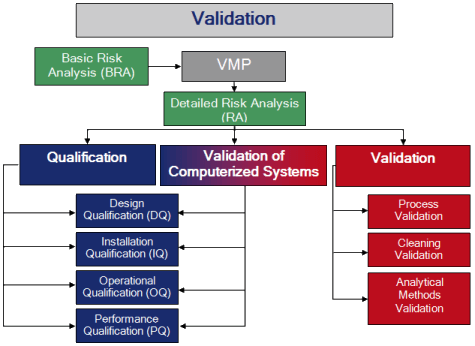
Our complete CQV qualification package includes hardware ( URS,RA,DQ,DS,FAT,SAT,CPT, IQ, OQ & PQ as well as CSV validation as per GAMP 5 (21-CFR-11 Compliance).
Our Approach for CSV as part of CQV process is as under.We perform CSV after OQ execution and before PQ.
Computerized system validation (CSV) is the documented process of assuring that a computerized system does exactly what it is designed to do in a consistent and reproducible manner.
CSV is of particular importance to industries requiring high-integrity systems that maintain compliant with current regulations designed by different national and international regulatory authorities like USFDA, EMEA, EU GMP, ANVISA Brazil, Health Canada, WHO and ICH Guidelines etc. under all circumstances.
Computer System Validation Methodology
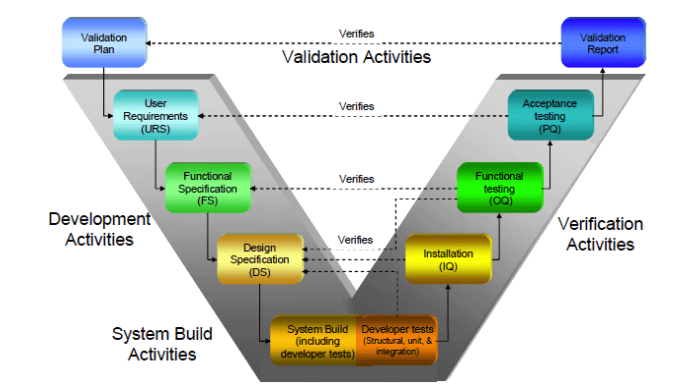
GAMP Services has developed a comprehensive solution to support Life Sciences companies with comprehensive testing of regulatory requirements. Our Validation Package provides assistance with core regulatory requirements.
Validation Process provides some pre-configured content rich set of templates, ready for customization to meet both domestic and international regulations. Our documentation templates include:
- Validation plan
- System Impact Assessment
- Requirements specifications
- System configuration specifications
- Test plan
- Testing and Reports for
- Installation Qualification (IQ)
- Operation Qualification (OQ)
- Performance Qualification (PQ)
- Assessment for compliance with regulations pertaining to electronic records and signatures (e.g., 21 CFR Part 11)
- Traceability matrix
- Quality assurance review
- Validation summary report
- Review of Standard Operating Procedures (SOPs) and Training



 Copyright GAMP Services 2024. All Rights Reserved.
Copyright GAMP Services 2024. All Rights Reserved.