
Facility BioDecontamination / FoggingService
BioDecontamination/Disinfectant fogging is a process of releasing a fine mist of disinfectant solution into the air of a cleanroom to sanitize all surfaces and reduce the risk of contamination.
The disinfectant solution is atomized using a specialized machine, creating a fog that spreads evenly throughout the room.Ensuring potential microbial contaminants are not transferred into cleanroom environments is a key aspect of risk mitigation strategies.
GAMP Group is a Partner of France.
GAMP Group has large pool of BioDecontamination Fogging System to provide the Biodecontamination system.
The minimum EU GMP Annex 1 requirement for transfer disinfection/decontamination is a transfer hatch with localised air supplies (Grade A / ISO class 5 at rest), also known as a dynamic pass-through hatch. Revised EU GMP Annex 1 highlights the importance of the transfer of materials, placing “an emphasis upon robust disinfection practices where the use of double-ended autoclaves is not possible.”


When is The Decontamination of Clean Rooms Required?
Biological contamination will occur, to some extent, in all types of cleanroom facility.
Whether a small quality control lab or a large vaccine production facility the control of microbiological contamination is essential.
If cleanliness is compromised, clean room decontamination needs to happen quickly and efficiently.
More often than not biological contamination will be introduced to the clean room by the people working within it.
This will include the direct contact made with surfaces or from respiratory aerosols or the shedding of hair and skin particles.
Biological contamination may also occur from the leakage of service pipework, damaged air filters, poorly maintained ventilation plant or loss of containment during power or plant outages.
Persistently high viable counts observed during routine monitoring may be the first warning of a problem.
Ideally the source of the contamination should be identified before proceeding with a room bio-decontamination service such that targeted remedial works or a change in procedure provide confidence the situation won’t reoccur.
If a facility has been shut down for routine maintenance, modification or refurbishment then biological decontamination will be necessary. When selecting the most appropriate decontamination method the following factors will require consideration :
- Efficacy : Consistent and robust performance against all types of organism including those considered difficult to deactivate (such as TB and Anthrax) is essential.
- Fully Documented : Detailed, professionally written reports ensuring traceability of the procedure and validation of the process are essential to ensure compliance.
- Speed : Fast decontamination cycles reduce plant downtime and minimise operating costs.
- Materials Compatibility : Some decontamination methods attack plastics and painted surfaces leaving long term damage.
GAMP Group has ventilation experts and our knowledge of HVAC plant ensures decontamination can be performed safely, quickly and effectively.
Devea,France equipment’s can be deployed within a day and has the fastest cycle times available.
Clean Room Decontamination
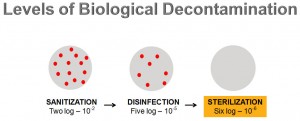
There are three levels of biological decontamination
- Sanitisation (making your area and the objects within it clean),
- Disinfection (eliminating pathogenic organisms, killing or making them harmless) and -sterilisation (completely eliminating microbial viability).
Offering different levels of sterility assurance, every log reduction represents a 90% reduction in microbial population, including non-pathogenic and pathogenic spores, fungi and viruses. In this way, a two log (10-2) reduction will offer your sanitisation, five logs (10-5) offers disinfection, while a six log (10-6) is chosen to reduce microbial population to close to zero – sterilisation.
Effective against a wide range of viral and fungal agents, and on a wide range of surfaces commonly found in clean rooms, such as glass and stainless steel, six log reduction is used for the decontamination of clean rooms.
At GAMP Group, we also provide decontamination services for equipment, entire buildings and modes of transport and our six log reduction can be used for the decontamination of other areas, including :
- Freeze Dryers
- Isolators and Incubators
- Containment Laboratories
- Robotic Enclosures
- Safety Cabinets
- Materials Transfer Devices
- Cold Rooms
- Food Production Facilities
GAMP Group’s Clean Room Decontamination Service
Working with our clients either on an ad hoc basis, or via a contract arrangement based on their ongoing decontamination requirements, at GAMP Group we use the latest revolutionary Microdrop technology of M/s Devea, France to deliver fast and effective decontamination services while also offering one of the most environmentally friendly solutions available.
The Microdrop technology of M/s Devea, France based technology has been proven effective in reducing microbial contaminants to below detectable levels, killing bacteria, viruses, spores and fungi including MRSA and C Difficile via a fine mist of hydrogen peroxide (6%) and isopropyl alcohol. The arc of mist reaches even into the less easy to access areas of your clean room environment, sterilising all exposed surfaces simultaneously.
This simultaneous clean means that our fast response times are enhanced further, and your clean room can be ready for use in as little as two hours, minimising your business down time while offering you peace of mind that your clean room standards have been restored via our verification process.



 Copyright GAMP Services 2024. All Rights Reserved.
Copyright GAMP Services 2024. All Rights Reserved.