
QMS Review & Development
Implementing a medical device quality management system, or QMS, is a regulatory requirement for medical device manufacturers. In the medical device industry, there is a strong focus on the regulatory requirements and creating conforming procedures. But a QMS should also make the organisation efficient and result in products that have high quality.
A Quality Management System, or QMS, is a comprehensive framework, or set of documented procedures, that guides people in an organisation to consistently deliver products that meet customer and regulatory requirements.
According to the ISO 9000:2015 standard, a Quality management system is defined as:
Quality Management System
a system to direct and control an organisation in terms of quality.
Importance of Quality Management System
For a medical device manufacturer, implementing a QMS based on the ISO 13485 standard will cover a lot of what is required, but it is not sufficient in itself.
The norms and standards that the medical device quality management system must meet depend on the type of medical device and which market the device is to be placed on:
- For the EU market
- 2017/745 – The Medical Device Regulation (MDR), or
- 2017/746 – The In-Vitro Diagnostic Medical Device Regulation (IVDR) (MDR), or
- ISO 13485 – Quality management systems – Requirements for regulatory purposes
- For the US market
- 21 CFR 820 – The Quality System Regulation
There may be other norms that have to be implemented in the QMS, for example:
- 2016/679 – General Data Protection Regulation (GDPR)
- Health Insurance Portability and Accountability Act of 1996 (HIPAA)
There are also voluntary standards that may be considered, for example:
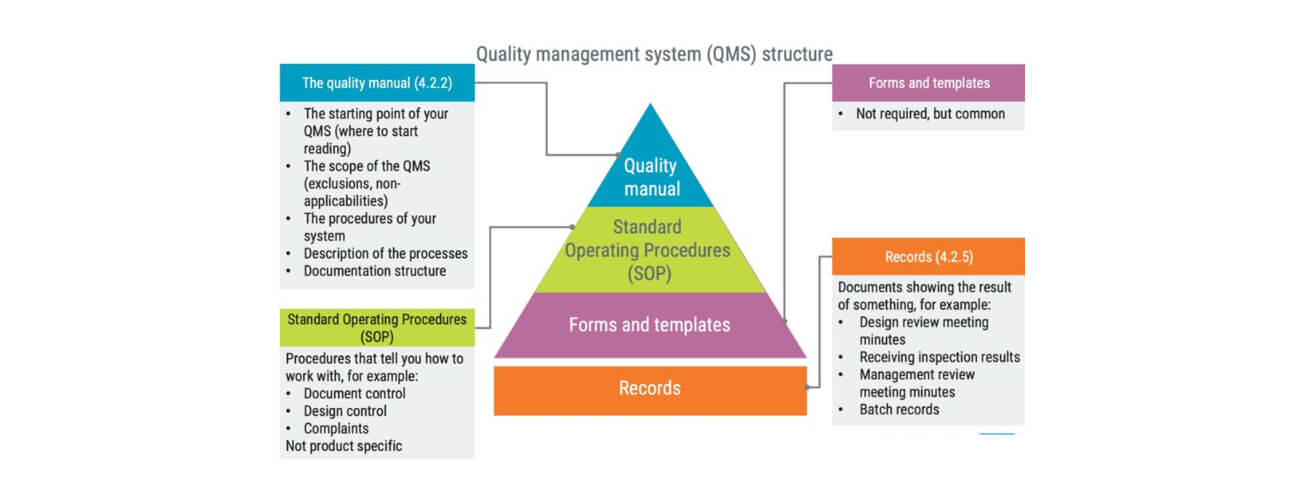
The structure of a medical device QMS
The key aims of the Quality Management Review for medical devices are:
To ensure consistent compliance with customer and regulatory requirements.
To improve QMS effectiveness and efficiency on a continuous basis. This is key, as the QMS sets the foundation for making medical devices that are available to end users, safe and effective.
To highlight emerging trends within the company. These can range from manufacturing (e.g., non-conforming products) and post market activities (feedback, complaints, PMCF etc.), to internal quality processes (e.g., failure to follow development processes, hence potential to deliver defective devices to market).



 Copyright GAMP Services 2024. All Rights Reserved.
Copyright GAMP Services 2024. All Rights Reserved.