Compressed Air / Nitrogen Gas validation
Compressed Air validation is a critical component in the production of pharmaceutical industry and effects on the quality of the end product. The Quality of Compressed air is important to ensure that product is safe. Compressed air validation is performed as per ISO 8573(Compressed Air – Contaminants and purity classes).
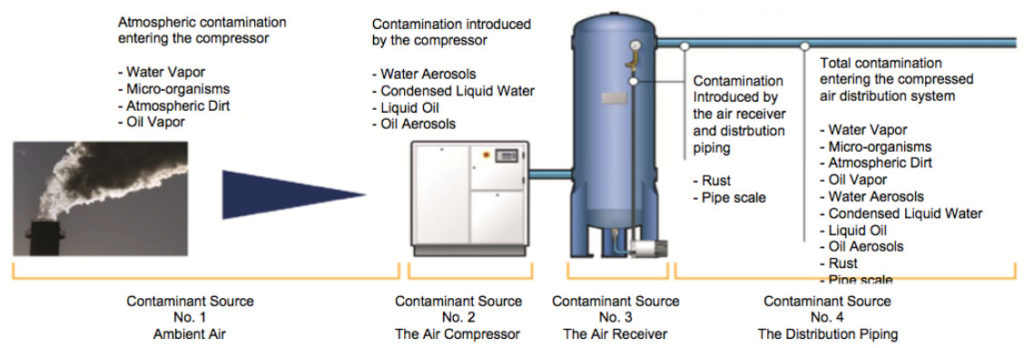
Without proper treatment, contaminants can travel from ambient air all the way through the compressed air system.
GAMP Services qualify Compressed air system with respect to contaminants of particles, water, oil, gases and microbiological contaminants.

The list of tests carried out under Compressed Air validation.
- Particle Count using HPD
- Microbial particle count
- Pressure Dew Point test
- Water vapour test (Moisture content)
- Odour Test
- Appearance test
- Carbon Dioxide
- Carbon Monoxide
- Nitrogen Monoxide and Nitrogen Dioxide
- Sulphur Dioxide
- Assay content of oxygen.
- Nitrogen purity test.
- Oil mist
- Steam Quality Test.
Steam quality testing is critical when performing any steam sterilization activity with an air removal step, such as autoclaving equipment.
International regulatory standards such as EN 285, EN 17665 & HTM2010 recommends steam quality testing and has defined parameters for compliance of clean steam. It is defined as the steam whose condensate meets all parameters of WFI. It must meet the basic physical properties as under.
GAMP Services performs Steam quality test using steam quality test kit.
