Computerized system validation (CSV) is the documented process of assuring that a computerized system does exactly what it is designed to do in a consistent and reproducible manner.
GAMP Services simple and appropriate manner of establishing the required documented evidence for the proof of the same holds a great value and assures that a computerized system will consistently perform as intended in its operational environment. The validation process begins with the system proposal/requirements definition and continues until system retirement and retention of the e-records based on regulatory rules.
CSV is of particular importance to industries requiring high-integrity systems that maintain compliance with current regulations designed by different national and international regulatory authorities like USFDA, EMEA, EU GMP, ANVISA Brazil, Health Canada, WHO and ICH Guidelines etc. under all circumstances.
GAMP Services cover the entire spectrum of Validation, Regulatory Compliance, and Audit Services.
We Provide Services for
- CSV- PLC (Control System) and Laboratory Instrument
- IT infrastructure
- Cloud Validation
- ERP and SAP Validation,
- LIMS Validation,
- Database backup, recovery and migration services
- Data Integrity Risk Assessment
- Pre and Post CSV Audit Services
- Design Qualification
- Conducting Vendors Audits, FAT (factory acceptance testing), and SAT (site acceptance testing)
- Software Developer Assessment
- Specifications—User Requirements, Functional Requirements, and Detailed Design
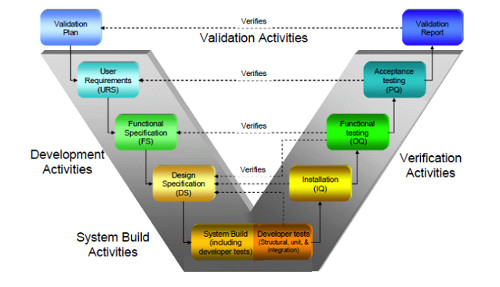
Computer System Validation Methodology
GAMP Services has developed a comprehensive solution to support Life Sciences companies with comprehensive testing of regulatory requirements. Our Validation Package provides assistance with core regulatory requirements.
⦁ The validation Process provides some pre-configured content-rich set of templates, ready for customization to meet both domestic and international regulations. Our documentation templates include:
- Validation plan
- System Impact Assessment
- Requirements specifications
- System configuration specifications
- Test plan
- Testing and Reports for
- Installation Qualification (IQ)
- Operation Qualification (OQ)
- Performance Qualification (PQ)
- Assessment for compliance with regulations pertaining to electronic records and signatures (e.g., 21 CFR Part 11)
- Traceability matrix
- Quality assurance review
- Validation summary report
- Review of Standard Operating Procedures (SOPs) and Training
