GAMP Services is one of the leading CQV (Commissioning, Qualification, and Validation) services provider globally for the entire life science segment includes pharmaceutical, medical device and food industry.
Core Focus-Sterile Facilities
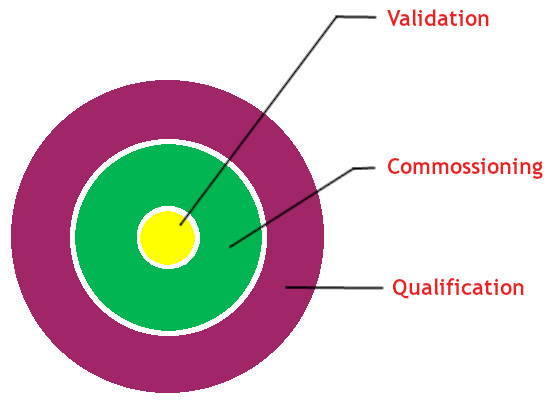
- Our team consists of experts & SMEs in the validation of facilities, utilities, equipment, computer systems & processes.
- We guide companies in selection of right Validation strategy and fully support from protocol preparation to execution to data analysis.
- Our team of CQC experts can support your company in the full CQV lifecycle as well as save your time and money during execution of the project.
- ISPE Baseline® Guide Volume 5: Commissioning & Qualification
- Impact Assessment
- Good engineering practice (GEP)
- Design reviews
- Commissioning
- Qualification (IQ, OQ and PQ)
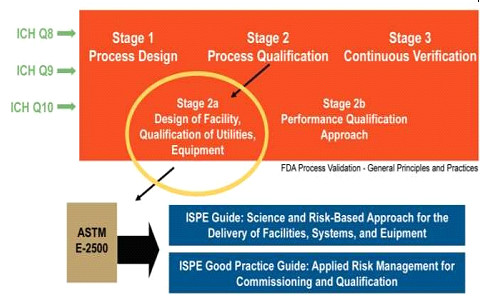
- ASTM E2500: Standard Guide for Specification, Design, and Verification of Pharmaceutical and Biopharmaceutical Manufacturing Systems and Equipment.
- Risk‐based and science‐based approach.
- Subject Matter Experts (SMEs) to drive the specification, design, and verification.
- Risk assessments to determine verification needs
- Verification list
- Verification test matrix
C &Q Approach
ASTM 2500 & ISPE BASELINE & GEP (Good Engineering Practice)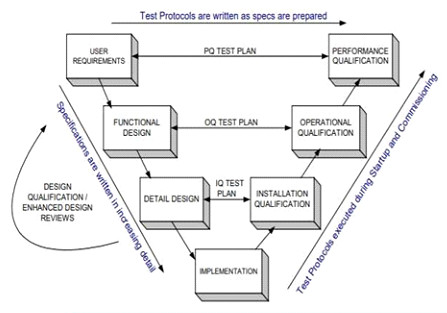
Our Expertise
- Facilities Equipment
- Utilities Equipment
- Greenfield CQV Projects
- New Production Suite Commissioning
- Decommissioning
Total CQV Lifecycle And Documentation:
- Project Planning
- Validation Master Plans (VMP)
- User Requirements Specifications (URS)
- Risk Assessments
- Protocols
- FAT
- SAT
- Summary Reports
- Traceability Matrixes
- Calibration Matrix
- SOPs
- Integrated Commissioning and Qualification (ICQ)
- Validation Protocol Format
- Design Qualification (DQ)
- Installation Qualification (IQ)
- Operational Qualification (OQ)
- Performance Qualification (PQ)
- Good Automated Manufacturing Practice (GAMP)
- Current Good Manufacturing Practice (cGMP) Review
- Continued Process Verification (CPV)
- Pre-Operational Verifications
