- Process validation is the verification that a process meets the requirements imposed on its process results. Learn when you must validate which processes (in the context of software) and how to ace validation. Furthermore, find out what process validation has to do with PQ, IQ, and OQ.
- What Is Process Validation
- Regulatory Requirements
- Practical Advice on Process Validation
Process Validation: What Exactly Is It?
a) Definition of the term “validation”
In general, validation is the “confirmation, through the provision of objective evidence, that the requirements for a specific intended use or application have been fulfilled” [ISO 9001:2015].
The standard remarks that the objective evidence necessary for validation is the result of a test or of another type of determination such as, for example, alternative calculations.
b) Definition of the term “process”
As for the definition of the term “process”, ISO 13485, again, refers to ISO 9000:2015. This norm conceives a process to be a “set of interrelated or interacting activities that use inputs to deliver an intended result.”
Example processes are:
- development process
- sterilization process
- production process
- recruitment process
- sales process
We should further note that a process not only requires inputs (“entries”) and outputs (“results”), but also resources such as humans or machines (including software / IT).
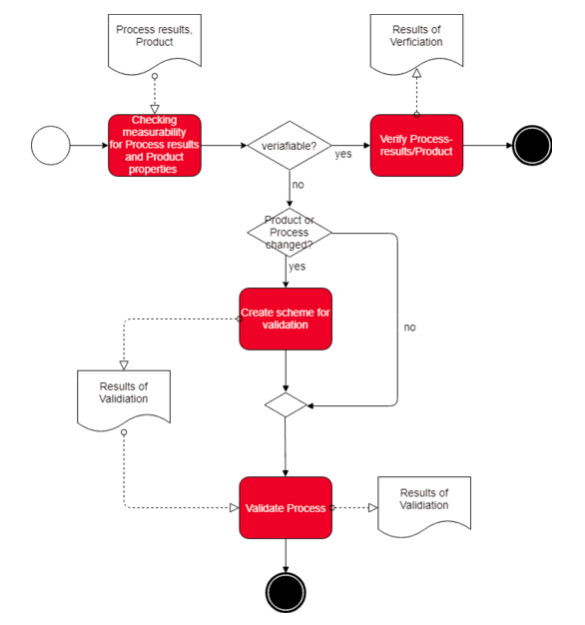
c) Process validation
If we combine both definitions, it becomes clear that a process validation provides a confirmation of a process leading to the intended process results by objective evidence
For instance, in case of a development process one would ascertain that the development outcomes meet the requirements (“Design Input”). As for a sterilization process, one would ensure that the good to be sterilized actually is sterile.
The FDA explicitly defines the term: “process validation means establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications. “
Process validation and PQ, IQ and OQ
Often, companies (especially in the pharmaceuticals sector) differentiate the following phases of process validation:
- IQ: This first inspections at the site of the customer shall ensure that the device was delivered, installed, and built up according to specifications, that the device meets the users’ requirements, and that the documentation is present. At this stage, the basis for calibration, maintenance, and cleaning is provided. It is advised to have a look at the manual :-).
- OQ: During this most extensive testing, it should be checked whether the device operates according to specifications, especially at specification limits, to know what might happen in the worst case.
- PQ, then, is only proof that the process runs under normal conditions. This test addresses, inter alia, measurement accuracy (incl. calibration and adjustment). It is also about long-term stability. In the pharmaceutical world, at least 3 batches are tested. A P-Diagram shows influencing factors such as environmental conditions, work steps, process parameters, and material properties. Here, sampling plans come into play.